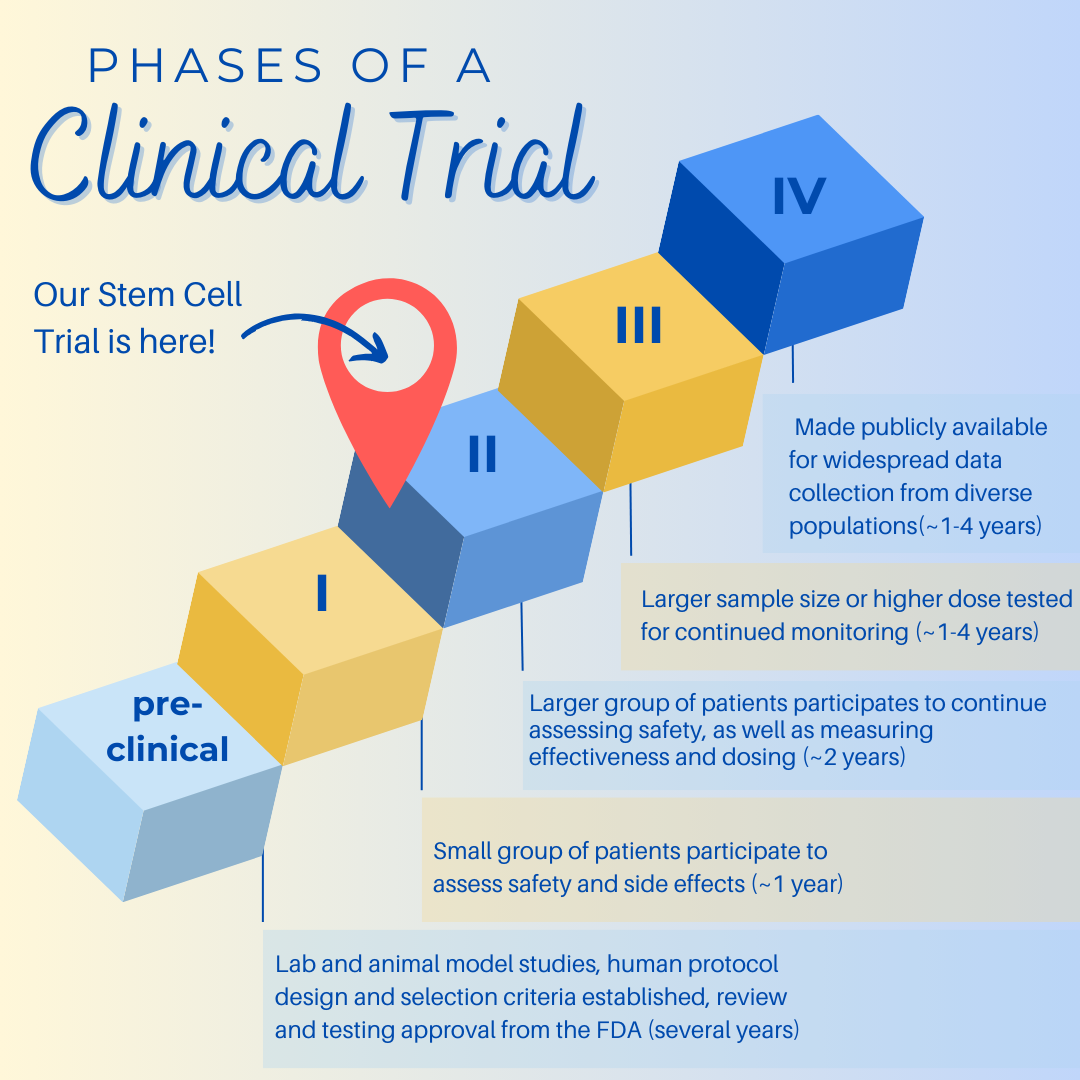
Explaining the Phases of Clinical Trial
Clinical trials are conducted in a series of steps, called phases - each phase is designed to answer a separate research question. All FDA-approved treatments must go through the clinical trial process to prove their safety and efficacy.
- Phase I: Researchers test a new drug or treatment in a small group of people for the first time to evaluate its safety, determine a safe dosage range, and identify side effects.
- Phase II: The drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
- Phase III: The drug or treatment is given to large groups of people to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely.
- Phase IV: Studies are done after the drug or treatment has been marketed to gather information on the drug's effect in various populations and any side effects associated with long-term use.
At Tisch MSRCNY, we have published the results of our Phase II trial, and are now in review with the FDA on next steps.
Additional Resource Information on clinical trials can be found at https://www.clinicaltrials.gov/ct2/resources